Using Your Grpah Describe the Change in Conductivity
For facts physical properties chemical properties structure and atomic properties of the specific element click. What kind of mathematical relationship does there appear to be between conductivity and concentration.
Linear or curved same or different slopes do the lines pass through the origin.
. Describe the appearance of each of the three curves on your graph in Step 14. When this increase in conductivity occurs let the titration proceed for several. The general equation for the plot is given as.
NaCl AlCl 3 and CaCl 2 4. You may make 3 separate plots or combine them all in one plot. Describe the appearance of each of the three curves on your graph.
The specific conductivity or conductivity of an electrolytic solution at any given concentration is the conductance of the unit volume of the solution. Conductivity decreases with a decrease in concentration as the number of ions per unit. Describe the appearance of each of the three curves on your graph.
Students should make sure that the probe is high enough for the beaker to fit under the probe. CONDUCTIVITY INDICATOR Measures a solutions conductivity degree Gives results in micromhos DIRECTIONS FOR USE. They should use an electrode support to suspend the conductivity probe from the ring stand.
As the concentration increased with the addition of drops so did the conductivity of the solution. Select CURVE FIT from the ANALYZE OPTIONS menu. Enter the Name Volume and Units drops.
DATA TABLE Solution Slope m 10 M NaCl 10 M AlCl 3 10 M CaCl 2 PROCESSING THE DATA 1. Press then select ANALYZE from the main screen. Where -A is a constant equal to the slope of the line.
Use the alligator clips to expand the circuit. High melting and boiling points. What kind of mathematical.
Be sure to label them properly. Describe the change in conductivity as the concentration of the NaCl solution was increased by the addition of NaCl drops. Construct a plot of conductivity vs.
Describe the change in conductivity as the concentration of the NaCl solution was increased by the addition of NaCl drops. On the Meter screen tap Mode. Set up the data-collection mode.
Describe the change in conductivity as the concentration of the NaCl solution was increased by the addition of NaCl drops. Please use proper grammar spelling and mechanics. Describe the change in conductivity as the concentration of the NaCl solution was increased by the.
Solution Slope m 10 M NaCl 10 M AlCl3 10 M CaCl2 PROCESSING THE DATA. What kind of mathematical relationship does there appear to be between conductivity and concentration. 10 M CaCl 2.
Describe the change in conductivity as the salt concentration was increased by the addition of more drops. Submerge the electrode in solution Stir for a few seconds and see the results. Describe the change in conductivity as the concentration of the NaCl solution was increased by the addition of NaCl drops.
Λm Λm AC½. Ʌm Ëm -Ac12. Describe the change in conductivity as the concentration of the NaCl solution was increased by the addition of NaCl.
Describe the mathematical relationship that. It is the conductance when kept between two platinum electrodes with a unit area of cross-section. Write at least 2 sentences about each.
Write the ions in each compound. The value of limiting molar conductivity Ëm can be determined from the graph or with the help of Kohlrausch law. Counter slot check the graph to see that the first data pair was recorded.
9 Conductivity is linked to temperature 9 Small variations 50 to 100 micromhos. Describe the change in conductivity as the concentration of the NaCl solution in the stirred beaker was increased by the addition of NaCl. In the below periodic table you can see the trend of Electrical Conductivity.
Expand your LED and battery circuit to create a conductivity meter. If you have an older sensor that does not auto-ID manually set up the sensor. This will be the equivalence point of the reaction.
Use the paper clips as the final electrodes that will be put into solution. Describe the appearance of each of the three curves on your graph eg. Where A is a constant and Λ is called molar conductivity at infinite dilutionThis equation is called Debye Huckel Onsager equation and is.
Enter your names and the number of copies of the graph you want. Select LINEAR CH 1 VS ENTRY from the CURVE FIT menu. Then plot the linear regression curve on your graph.
To analyze the relationship between conductivity and volume use this method to calculate the linear-regression statistics for your data. This problem has been solved. Continue watching your graph to see when the conductivity begins to increase.
HOW TO MEASURE CONDUCTIVITY. For a given solvent the value of A depends on the type of electrolyte at a particular temperature. The electrodes are at a distance of unit length.
Using Excel make a plot of your conductivity y-axis vs volume x-axisdata for NaCl Na 2 SO 3 and BaCl 2. The variation of molar conductivity with concentration may be given by the expression. Students should connect the conductivity probe to the EasyLink or GoLink interface and then connect the interface to their handheld devices or computers.
Test your circuit by touching the two paper clip electrodes together to light the LED. Is there a direct or inverse proportion between conductivity and concentration. Describe each of the following 5 properties of metals.
Use three list and two stat plots for two experiments. Describe the appearance of the curve on your graph. Connect the Conductivity Probe to LabQuest and choose New from the File menu.
For each graph perform a linear regression and be sure to include the equation of the straight line on the plot. Change the mode to Events with Entry. Describe the appearance of each of the three curves on your graph.
DATA TABLE Get the data from the graphs slope is boxed make sure to include all of the units µS is micro Sieverts Solution. Up to 24 cash back Enter your names and the number of copies of the graph you want. Periodic Table of Elements with Electrical Conductivity Trends.
Describe the change in conductivity as the concentration of the NaCl solution was increased by the addition of NaCl drops. Observe that the conductivity readings decrease gradually. Describe the appearance of each of the three curves on your graph.
NaCl concentration or NaCl volume in drops using your data.

Variation Of Electrical Conductivity Of Distilled Water As A Function Download Scientific Diagram

Variation Of Electrical Conductivity Of Distilled Water As A Function Download Scientific Diagram

Conductivity Curves In Titrations Youtube

Relationship Between Electrical Conductivity Of The Detergent Solution Download Scientific Diagram
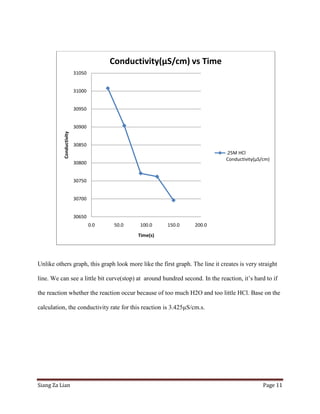
Comments
Post a Comment